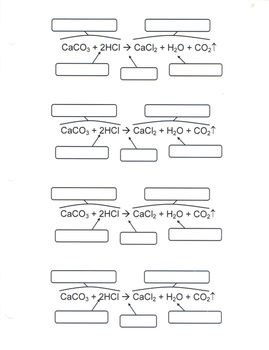
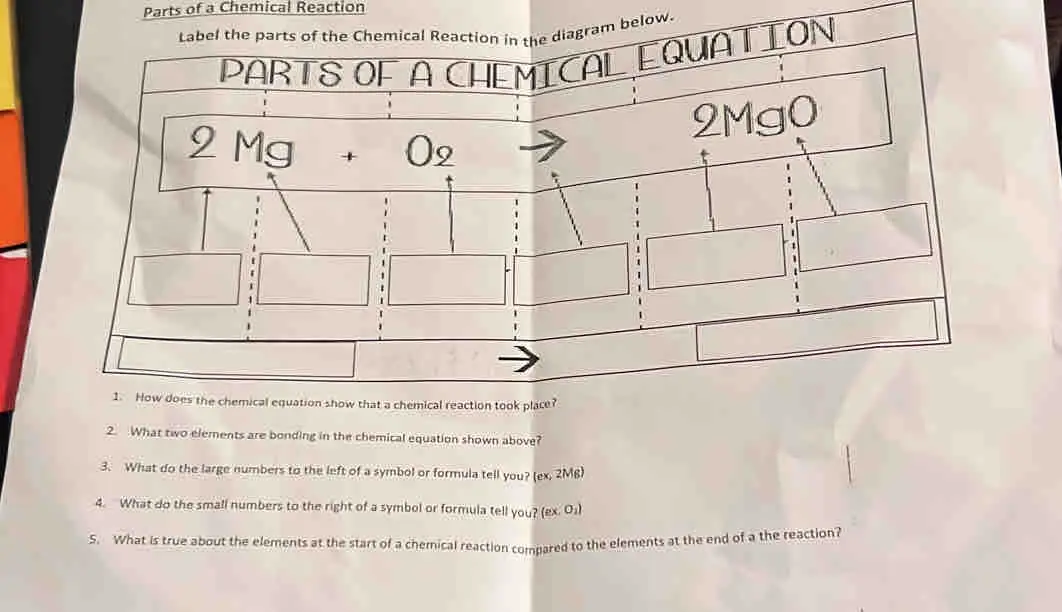
40 label the parts of a chemical reaction
2.17: Reactants and Products - Chemistry LibreTexts In words, we could write the reaction as: zinc + sulfur → zinc sulfide The more convenient way to express a chemical reaction is to use the symbols and formulas of the substances involved: Zn + S → ZnS The substance (s) to the left of the arrow in a chemical equation are called reactants. Chemistry Worksheets and Handouts (PDF for Printing) Print free chemistry worksheets and handouts to enhance student learning. This is a collection of free chemistry worksheets and handouts to print. Most of the printables are PDF files, although some are available as JPG or PNG files. All of these worksheets print cleanly on normal printer paper, plus you can resize them to fit your needs.
What Are The Three Main Parts Of A Chemical Reaction - BYJU'S What Are The Three Main Parts Of A Chemical Reaction We will look at the three main parts of a chemical reaction. Find out their names and their significance and how they are represented in an equation. Login Study Materials NCERT Solutions NCERT Solutions For Class 12 NCERT Solutions For Class 12 Physics NCERT Solutions For Class 12 Chemistry

Label the parts of a chemical reaction
ATP cycle and reaction coupling | Energy (article) | Khan Academy When two reactions are coupled, they can be added together to give an overall reaction, and the ΔG of this reaction will be the sum of the ΔG values of the individual reactions. As long as the overall ΔG is negative, both reactions can take place. Even a very endergonic reaction can occur if it is paired with a very exergonic one (such as ... What are the Parts of a Chemical Equation? | Life Persona What are the Parts of a Chemical Equation? Basically there are three Main parts in a chemical equation : The reactants, the products and the arrow indicating the direction of the chemical reaction. A chemical equation is an abbreviated form of representing the components of a chemical reaction. Enzymes and the active site (article) | Khan Academy A substance that speeds up a chemical reaction—without being a reactant—is called a catalyst. The catalysts for biochemical reactions that happen in living organisms are called enzymes. Enzymes are usually proteins, though some ribonucleic acid (RNA) molecules act as enzymes too.
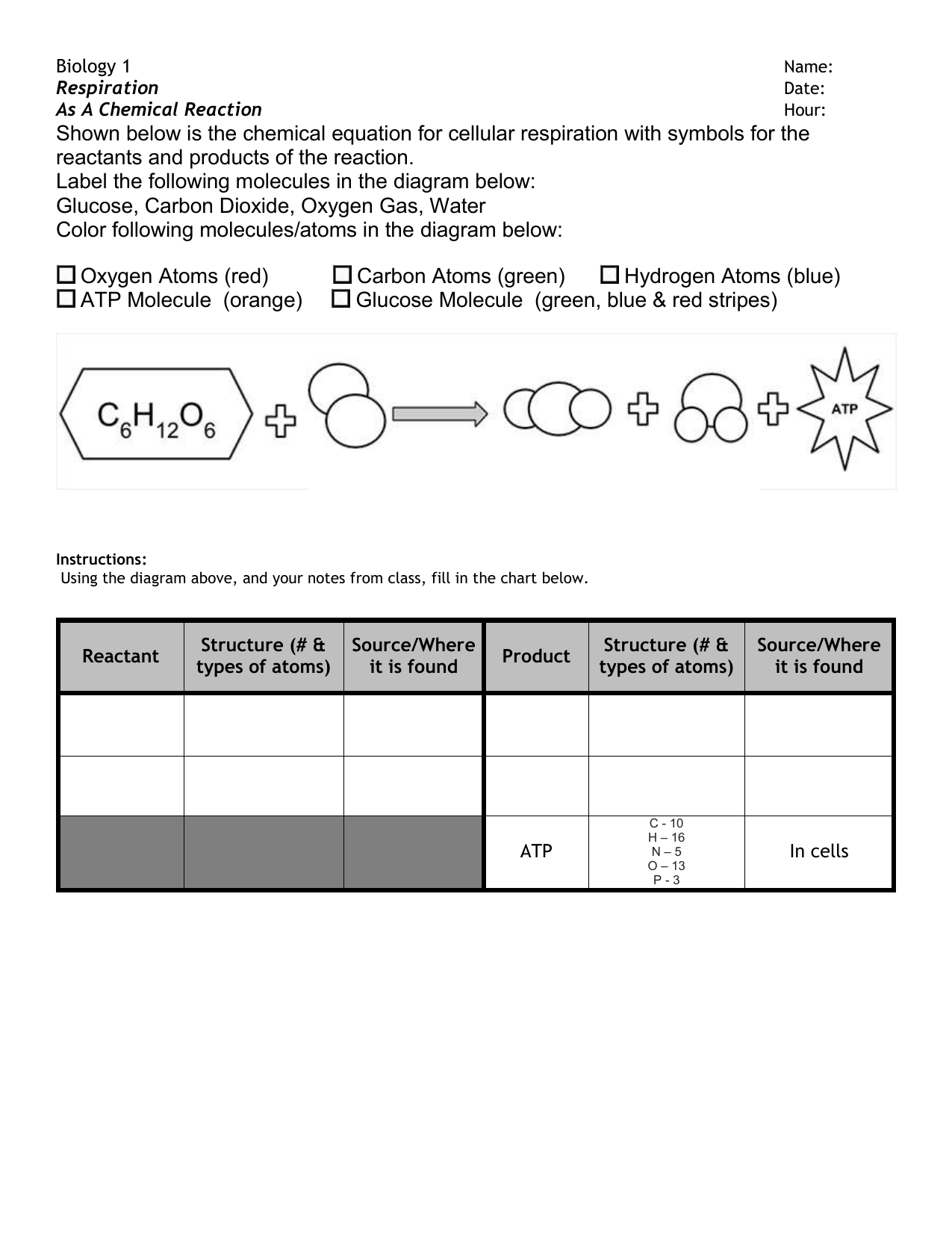
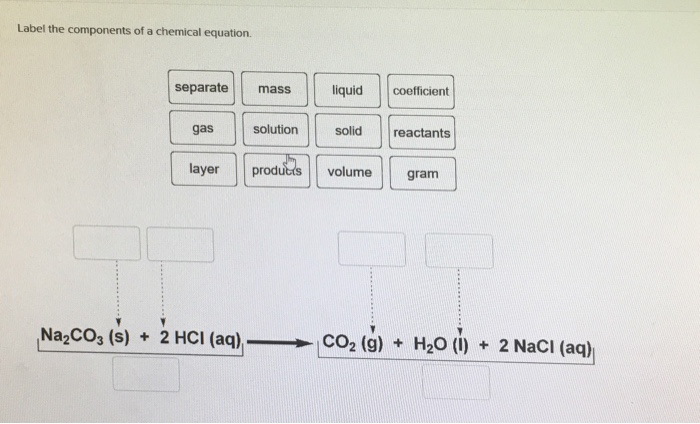
Label the parts of a chemical reaction. Parts of a Chemical Reaction Flashcards | Quizlet Substances at the beginning of a reaction. Products Substances at the end of a reaction. Coefficients Number in front of the formula that shows how many molecules in that formula. Subscripts Number after an element that shows how many molecules in that specific element. Arrow "Turns into" or "yields". Law of Conservation of Mass 7.4: How to Write Balanced Chemical Equations If a fractional coefficient has been used, multiply both sides of the equation by the denominator to obtain whole numbers for the coefficients. Count the numbers of atoms of each kind on both sides of the equation to be sure that the chemical equation is balanced. Example 7.4. 1: Combustion of Heptane. Chemical reactions | Chemistry of life | Biology (article) - Khan Academy Chemical reactions occur when chemical bonds between atoms are formed or broken. The substances that go into a chemical reaction are called the reactants, and the substances produced at the end of the reaction are known as the products.An arrow is drawn between the reactants and products to indicate the direction of the chemical reaction, though a chemical reaction is not always a "one-way ... 3.10: Writing and Balancing Chemical Equations A chemical reaction represents a change in the distribution of atoms, but not in the number of atoms. In this reaction, and in most chemical reactions, bonds are broken in the reactants (here, Cr-O and N-H bonds), and new bonds are formed to create the products (here, O-H and N≡N bonds).
Photosynthesis | Definition, Formula, Process, Diagram, Reactants ... In chemical terms, photosynthesis is a light-energized oxidation-reduction process. (Oxidation refers to the removal of electrons from a molecule; reduction refers to the gain of electrons by a molecule.) In plant photosynthesis, the energy of light is used to drive the oxidation of water (H 2 O), producing oxygen gas (O 2 ), hydrogen ions (H ... What is a Chemical Equation? - Definition & Examples A chemical equation provides information about the correct proportions of ingredients that are needed to make new substances in chemical reactions. To make a large glass of lemonade, use 1 1/2 ... Chemical Reaction - Chemistry Definition - ThoughtCo The general form of the reaction is: A + B → AB Decomposition Reaction A decomposition reaction is the reverse of a synthesis reaction. In a decomposition, a complex reactant breaks into simpler products. The general form of a decomposition reaction is: AB → A + B Single Replacement Reaction Double replacement reactions - Khan Academy Lesson 5: Types of chemical reactions. Oxidation-reduction (redox) reactions. Worked example: Using oxidation numbers to identify oxidation and reduction. ... Double replacement reactions—also called double displacement, exchange, or metathesis reactions—occur when parts of two ionic compounds are exchanged, making two new compounds. The ...
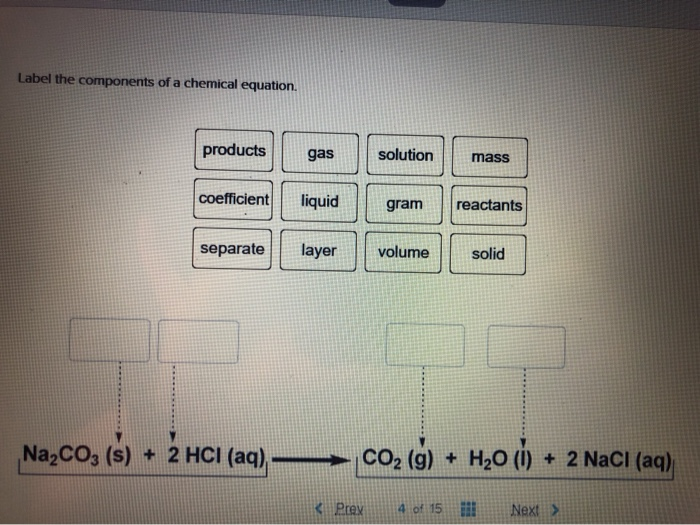
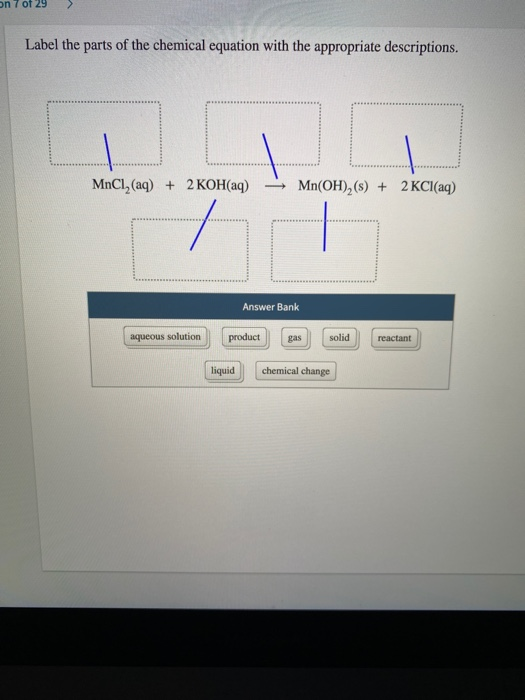
5.3: Types of Chemical Reactions - Chemistry LibreTexts The chemical reaction was a simple one: hydrogen combining with oxygen to produce water. Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. The products of the combustion of hydrocarbons are always carbon dioxide and water. Many hydrocarbons are used as fuel because their combustion releases ... 7.10: Classifying Chemical Reactions - Chemistry LibreTexts Most chemical reactions can be classified into one or more of five basic types: acid-base reactions, exchange reactions, condensation reactions (and the reverse, cleavage reactions), and oxidation-reduction reactions. The general forms of these five kinds of reactions are summarized in Table 7.10.1, along with examples of each. Label the parts of the chemical equation with the appropriate ... - Wyzant Label the parts of the chemical equation with the appropriate descriptions. Sr (NO3)2 (aq) + K2CO3 (aq) SrCO3 (s) + 2KNO3 (aq) answer bank- Product, Liquid, gas, reactant, solid, aqueous solution. Follow • 2 Add comment Report 1 Expert Answer Best Newest Oldest J.R. S. answered • 11/14/20 Tutor 5.0 (141) Chemical Reactions Overview - Chemistry LibreTexts Chemical reactions are the processes by which chemicals interact to form new chemicals with different compositions. Simply stated, a chemical reaction is the process where reactants are transformed into products. How chemicals react is dictated by the chemical properties of the element or compound- the ways in which a compound or element ...
Chemical reaction | Definition, Equations, Examples, & Types chemical reaction, a process in which one or more substances, the reactants, are converted to one or more different substances, the products. Substances are either chemical elements or compounds. A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products.
Enzyme structure and function (article) | Khan Academy Enzymes are the catalysts involved in biological chemical reactions. They are the "gnomes" inside each one of us that take molecules like nucleotides and align them together to create DNA, or amino acids to make proteins, to name two of thousands of such functions.
Enzymes and the active site (article) | Khan Academy A substance that speeds up a chemical reaction—without being a reactant—is called a catalyst. The catalysts for biochemical reactions that happen in living organisms are called enzymes. Enzymes are usually proteins, though some ribonucleic acid (RNA) molecules act as enzymes too.
What are the Parts of a Chemical Equation? | Life Persona What are the Parts of a Chemical Equation? Basically there are three Main parts in a chemical equation : The reactants, the products and the arrow indicating the direction of the chemical reaction. A chemical equation is an abbreviated form of representing the components of a chemical reaction.
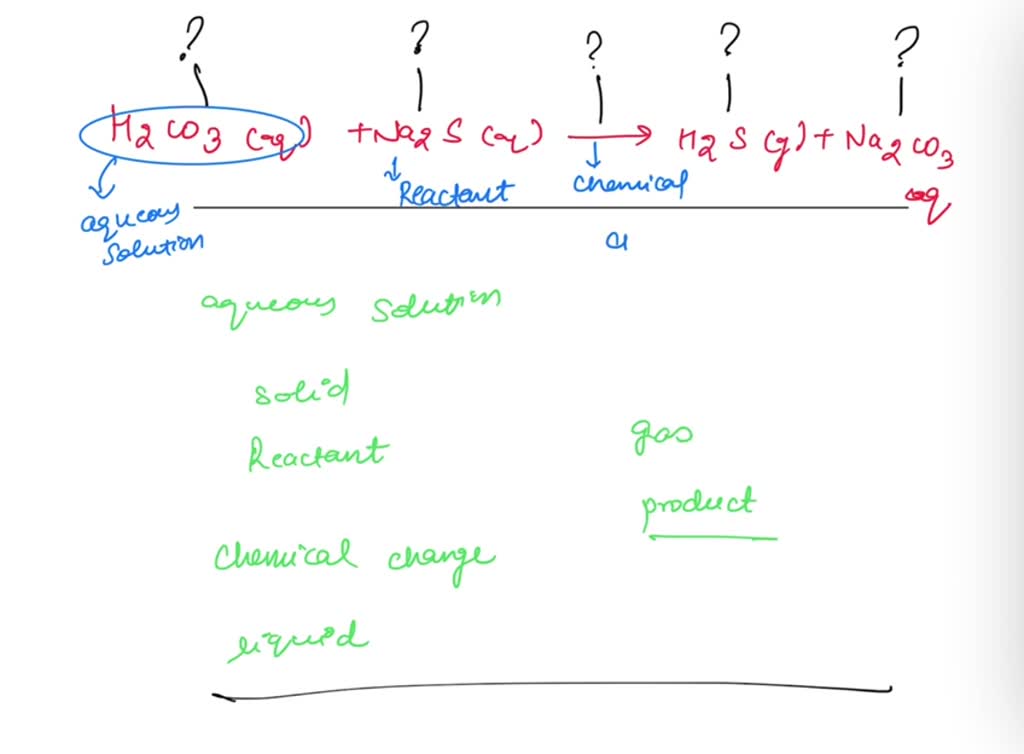
Label the parts of the chemical equation with the appropriate descriptions, HzCOz(aq), Naz S(aq), HzS(g), Naz COz(aq), Answer Bank, aqucous solution, solid, reactant, chemical change, liquid, product
ATP cycle and reaction coupling | Energy (article) | Khan Academy When two reactions are coupled, they can be added together to give an overall reaction, and the ΔG of this reaction will be the sum of the ΔG values of the individual reactions. As long as the overall ΔG is negative, both reactions can take place. Even a very endergonic reaction can occur if it is paired with a very exergonic one (such as ...






















Post a Comment for "40 label the parts of a chemical reaction"