40 which of the following foods is exempt from listing ingredients on the label
What are the Requirements for a Food Label? - Short Food Labeling Guide When labeling mixes of fruits or vegetable juices on the label, you must list the fruits and vegetables in descending order of prominence by volume. The ingredient list is an exception to this rule. And if the label indicates that the juices are only used for flavor, then this rule does not apply. Helpful Resources for Food Labeling What Is Bioengineered Food? New Laws, Bioengineered vs. GMO + Risks Exemptions to the new BE labeling include: foods that are, or are primarily made with, meat, poultry or egg products (these must be the first ingredients) or foods that are primarily made with water, broth or stock (again, these must be the first ingredient listed).
FDA Food Product Labeling & Packaging Requirements - ESHA The Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA) mandates that packaged food items must declare, in plain language, the presence of any major food allergens (Milk, Egg, Fish, Crustacean shellfish, Tree nuts, Wheat, Peanuts, Soybeans, Sesame) on the product packaging.

Which of the following foods is exempt from listing ingredients on the label
6 Things You Need to Know About the New GMO Food Label These foods may state "Derived From Bioengineering" or "Ingredients Derived From a Bioengineered Source" on their label. 4. Some Foods Are Exempt From the New BE Labeling Law. Products ... tax.iowa.gov › iowa-sales-tax-foodIowa Sales Tax on Food | Iowa Department of Revenue Certain food items incorporated into foods are exempt. An example is pectin, commonly used as a base in making jams and jellies. Other examples are lard and vegetable oils. Food Baskets. Food basket sales are exempt if the value of the exempt food items is greater than the value of the taxable items. CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Sec. 101.100 Food; exemptions from labeling. (a) The following foods are exempt from compliance with the requirements of section 403 (i) (2) of the act (requiring a declaration on the label of the...
Which of the following foods is exempt from listing ingredients on the label. WHMIS 2015 - Labels : OSH Answers - Canadian Centre for Occupational ... Supplemental label information - some supplemental label information is required based on the classification of the product. For example, the label for a mixture containing ingredients with unknown toxicity in amounts higher than or equal to 1% must include a statement indicating the percent of the ingredient or ingredients with unknown toxicity. Food and Product Regulations for the Farmers Market - SDSU Extension Ingredient statements should include all the ingredients in the recipe in order of predominance, by weight. The ingredient statement must include common names. The sub-ingredients of a food that is an ingredient in another food may be declared parenthetically following the name of the ingredient. Food labelling - general EU rules - Your Europe Labelling. Mandatory information must be printed using a font with a minimum x-height of 1.2 millimetres. If the largest surface area of packaging is less than 80 cm², you can use a minimum x-height of 0.9 mm. For packaging surface of less than 10 cm², you must list: name of the food. any substances or products causing allergies or ... Food products that require a label - Canadian Food Inspection Agency The FDR and SFCR exempt the following prepackaged foods from carrying a label: one-bite confections that are sold individually [B.01.003 (1) (a) (i), FDR; 213 (a), SFCR] When more than 1 individual one-bite confection are sold together in the same package, the product sold is not considered to be a one-bite confection.
Labelling requirements for fats and oils - Canadian Food Inspection Agency A number of fat and oil products, when used as ingredients in foods, are usually exempt from declaring components in the list of ingredients. These include margarine, shortening, lard, and, in certain situations, standardized oils. See Ingredients that generally do not have to declare their components for more information. Food Additive Status List | FDA N-Acetyl-L-methionine (free, hydrated, or anhydrous, or sodium or potassium salts) - NUTR, REG, In foods, except infant foods and foods containing added nitrites/nitrates -172.372 Acetylated... Active Ingredients Allowed in Minimum Risk Pesticide Products Active Ingredients Allowed in Minimum Risk Pesticide Products The following active ingredients can be used in pesticide products that are exempt from the requirements of the Federal Insecticide, Fungicide, and Rodenticide Act under the minimum risk exemption regulations in 40 CFR 152.25 (f). What foods have titanium dioxide? What to know after Skittles lawsuit The FDA first approved the use of titanium dioxide in food in 1966, following its 1960 removal (along with the removal of other color additives) from the agency's original "Generally Recognized as ...
Industry Systems FDA Industry Systems / FDA Unified Registration and Listing Systems (FURLS) / Technical Help. Electronic Submissions Gateway Approved Production Transaction Partners, Food Facility Registration Module, Low Acid & Acidified Canned Foods, and Account Management. Phone: 1-800-216-7331 or 240-247-8804 9:00 a.m.- 6:00 p.m. Eastern Time Prepacked for direct sale (PPDS) allergen ... - Food Standards Agency Examples of food that is prepacked for direct sale Foods that may be provided by a butcher or farm shop include: sausages, burgers, or other foods packaged in store before being ordered or... › business-guidance › packaging-andPackaging and labelling | Food Standards Agency Some foods are exempt from the need to display an ingredient list, for example: fresh fruit and vegetables, carbonated water and foods consisting of a single ingredient etc. For further information about the legal requirements that apply to naming the food and listing ingredients, please visit our Food Information Regulations 2014 summary guidance. FDA Labeling & Incidental Additives | Registrar The U.S. Food and Drug Administration's (FDA's) labeling regulations generally require that firms list all ingredients present in a food product on the product's label. However, certain circumstances can allow an exemption from this regulation. An example of a substance that may be exempt from labeling requirements is an incidental additive.
Inert Ingredients Overview and Guidance | US EPA Food and Nonfood Use - The only inert ingredients approved for use in pesticide products applied to food are those that have either tolerances or tolerance exemptions in the Code of Federal Regulations (CFR), 40 CFR part 180 (the majority are found in sections 180.910 - 960), or where no residues are found in food.
Iowa Sales Tax on Food The sale of these products is generally exempt from sales tax. Typical examples are nuts, potato chips, popcorn, corn chips, and pretzels. Snack food items are not taxable because they are eligible to be purchased with Food Stamps.
GMO Foods Will Be Labeled 'Bioengineered' - Verywell Health According to the USDA, "highly refined ingredients (like some sugars and oils) and foods that are primarily meat, poultry, or egg products, do not require a bioengineered food disclosure." 4 According to the Center for Food Safety (CFS), a vast majority of bioengineered foods fall under the "highly refined" category.
› CottageFoodLaws-NorthNorth Carolina Cottage Food Laws and Regulations: How to sell ... They currently provide one format of the label, the Nutrition Labeling and Education Act 1990 Nutrition Facts Panel format. As of 2020, the cost was $150 per item or sample. . Directions about how to create the ingredient statement: Step 1: List ingredients in descending order by weight.
en.wikipedia.org › wiki › Food_coloringFood coloring - Wikipedia The U.S. FDA's permitted colors are classified as subject to certification or exempt from certification in Code of Federal Regulations – Title 21 Part 73 & 74 Archived October 23, 2008, at the Wayback Machine, both of which are subject to rigorous safety standards prior to their approval and listing for use in foods.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Sec. 101.100 Food; exemptions from labeling. (a) The following foods are exempt from compliance with the requirements of section 403 (i) (2) of the act (requiring a declaration on the label of the...
Labeling Policies | Food Safety and Inspection Service Retail stores are welcome to download, print, display and/or distribute them to consumers in close proximity to the relevant foods in the stores. The list of food items with nutrition information reflects the updates published in the Federal Register of December 29, 2010. Chicken & Turkey Poster (30" x 38.5"; 5.22MB; PDF Only)
Food Labelling - CFS "Soybeans", "soy", "soya", "soya beans", "soy beans" or "Soyabeans" are all acceptable alternative names to designate "soyabeans" in the list of ingredients. The term " 黃 豆 " is commonly used in the trade and it is acceptable. 7. Is it necessary to label "Rice (contain gluten)" for the product of prepackaged rice?
Introduction to allergen labelling changes (PPDS) - Food Standards Agency From 1 October 2021, the requirements for prepacked for direct sale (PPDS) food labelling changed in Wales, England, and Northern Ireland. This labelling helps protect your consumers by providing potentially life-saving allergen information on the packaging. Any business that produces PPDS food is required to label it with the name of the food ...
Big 8 Food Allergies: Handling Allergens at Your Restaurant Otherwise, the allergen's food source name must be declared at least once on the food label in one of two ways: In parentheses: Following the name of the ingredient, list the allergen in parentheses. For example, "Lecithin (soy), Flour (wheat), and Whey (milk)."
› cfr › text9 CFR § 317.2 - Labels: definition; required features. Each component listing may utilize the required quantifying statement at the end of each component ingredients listing. (B) Such ingredients may be adjusted in the product formulation without a change being made in the ingredients statement on the labeling, provided that the adjusted amount complies with part 319 of this subchapter and with ...
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and...
How to Manufacture, Label, and Ship Food Products Like a Pro - OnlineLabels For the safety of consumers, food manufacturers must list all ingredients used to create their product. Of course, there are several exceptions to this requirement. For example, if you're selling something that's just one ingredient - like nuts or honey - there's no need to list ingredients on the label.
FNB News - Certified colours and colours exempt from certification ... The label must list the names of any FDA-certified color additive (e.g., FD&C Blue No. 1 or the abbreviated name, Blue 1). With the exception of carmine/cochineal extract, colour additives exempt from certification can be listed collectively as 'artificial colours', 'artificial colour added', 'colour added', or equally informative terms ...
Additives in food products - EU labelling rules - Your Europe EU assistance to Ukraine. Food additives sold by themselves or as ingredients in food products must follow strict EU rules. Additives come in a number of classes, including: sweeteners. preservatives. antioxidants. colours. In an ingredient list, most food additives and food enzymes must be preceded by the name of the category to which they ...
Nutrition Facts Labeling Services - Alabama Cooperative Extension System Insignificant amounts are instances where all ingredients round to zero or less than 1 gram. Some examples of this include coffee beans, tea, and some spices. Other exemptions include the following: Producers that employ less than 100 full-time employees and sell fewer than 100,000 units of the product per year.
Food labelling - Health.vic Food labels must carry essential information, so that consumers are informed of the nature and properties of foods before they buy. Food businesses must ensure that they do not mislead or deceive consumers with any claims made on food labels. Food importers must also comply with Australian labelling laws. All packaged foods sold in Australia ...
› en › health-canadaFood Allergen Labelling - Canada.ca The Food and Drug Regulations require that most prepackaged foods carry a label and that the ingredients appear on labels in decreasing order of proportion. However, some ingredients used in food products which were previously exempt from declaration in the list of ingredients, (e.g., components of margarine, seasoning and flour) will now be required to appear on food labels also.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Sec. 101.100 Food; exemptions from labeling. (a) The following foods are exempt from compliance with the requirements of section 403 (i) (2) of the act (requiring a declaration on the label of the...
tax.iowa.gov › iowa-sales-tax-foodIowa Sales Tax on Food | Iowa Department of Revenue Certain food items incorporated into foods are exempt. An example is pectin, commonly used as a base in making jams and jellies. Other examples are lard and vegetable oils. Food Baskets. Food basket sales are exempt if the value of the exempt food items is greater than the value of the taxable items.
6 Things You Need to Know About the New GMO Food Label These foods may state "Derived From Bioengineering" or "Ingredients Derived From a Bioengineered Source" on their label. 4. Some Foods Are Exempt From the New BE Labeling Law. Products ...

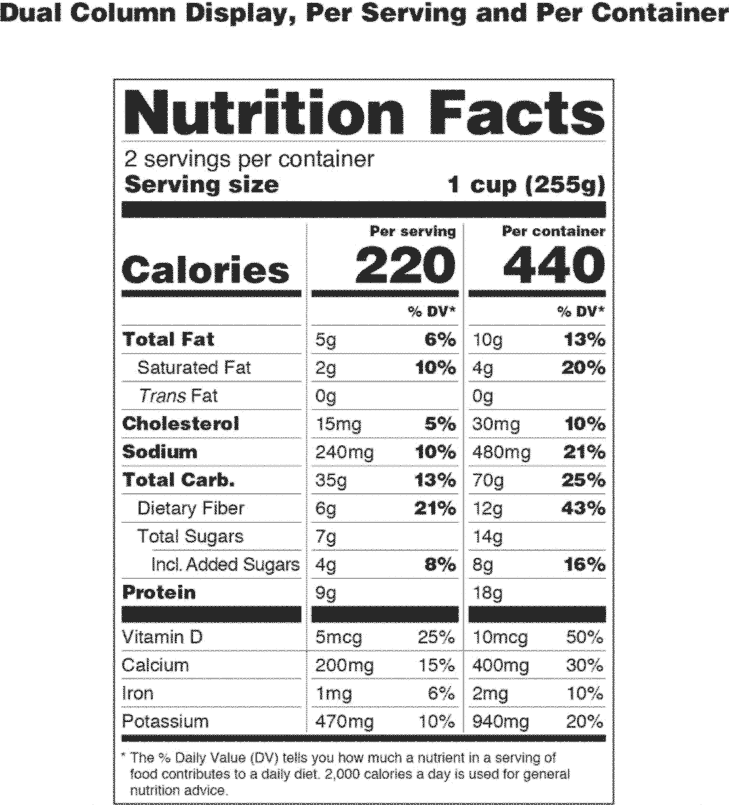









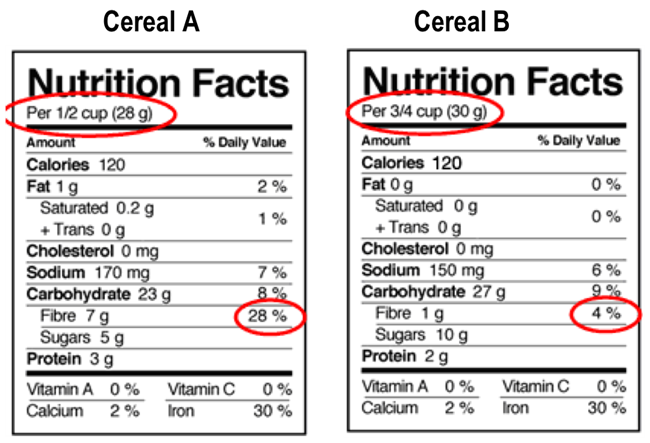










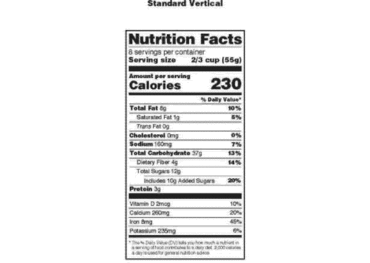





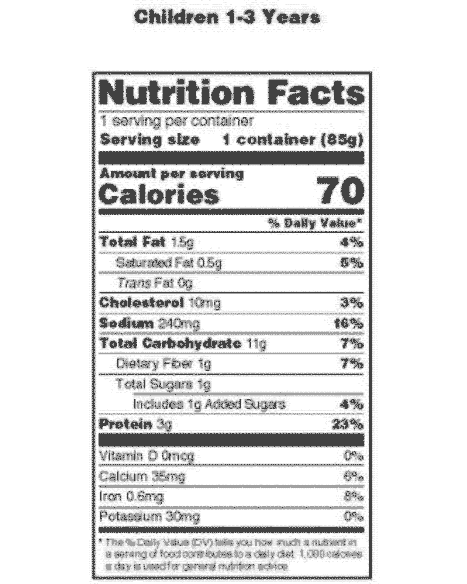
Post a Comment for "40 which of the following foods is exempt from listing ingredients on the label"