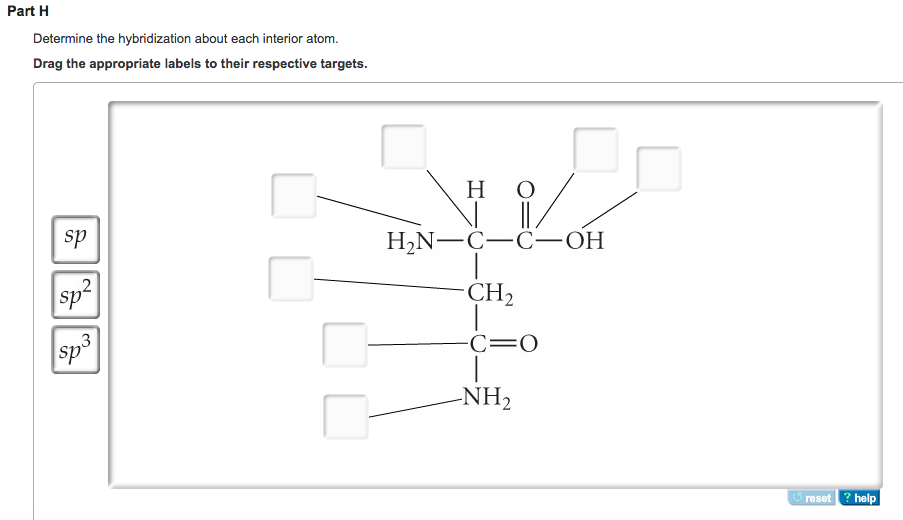
44 label each carbon atom with the appropriate hybridization.
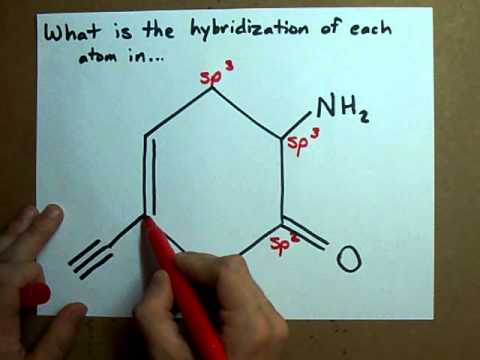
What is the hybridization of each carbon atom in ... This will account for. 4 × 2 e− +1 × 6 e− = 14 e−. The remaining 2 valence electrons will be added on the nitrogen atom as a lone pair. In order to find the hybridization of the two carbon atoms, you must count the regions of electron density that surround the atoms. A region of electron density is simply. a single, double, or triple bond. Hybridization of Carbon - Molecular Geometry and Bond Angles 2. sp2 Hybridization A carbon atom is sp2 hybridized when bonding takes place between 1 s-orbital with two p orbitals. There is a formation of two single bonds and one double bond between three atoms. The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds. Example: Hybridization of graphite 3. sp3 Hybridization
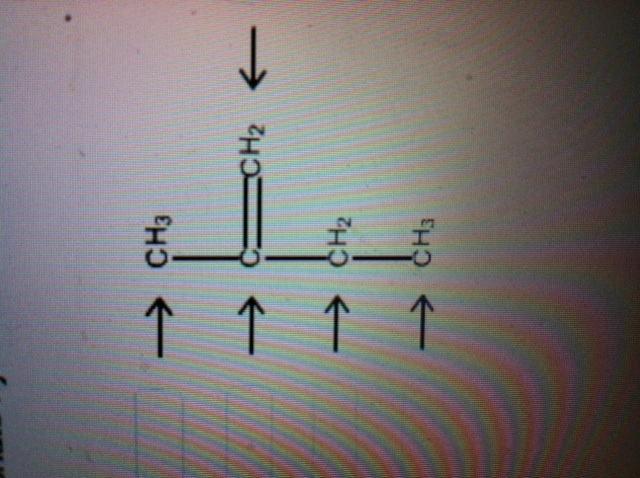
What orbitals are used to form the 10 sigma bonds in propane (ch3ch2ch3 ... The electrons of each carbon atom are found in one s-orbital and three p-orbitals. However, when forming sigma bonds, the carbon atoms combine the four atomic orbitals into four molecular orbitals. This results in each carbon now having four hydridized sp3 orbitals. Therefore, each carbon atom is sp3 hybridized.

Label each carbon atom with the appropriate hybridization.
Solved Label each carbon atom with the appropriate - Chegg Question: Label each carbon atom with the appropriate hybridization. This problem has been solved! ... Label each carbon atom with the appropriate hybridization. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Solved Label each carbon atom with the appropriate - Chegg Label each carbon atom with the appropriate hybridization. sp hybridized sp hybridized hybridized ; Question: Label each carbon atom with the appropriate hybridization. sp hybridized sp hybridized hybridized Solved Label each carbon atom with the appropriate - Chegg Question: Label each carbon atom with the appropriate hybridization. This problem has been solved! See the answerSee the answer ...
Label each carbon atom with the appropriate hybridization.. Answered: give the hybridization of each carbon… | bartleby Solution for give the hybridization of each carbon atom; close. Start your trial now! First week only $4.99! arrow_forward. learn. write. tutor. study resourcesexpand_more. Study Resources. We've got the study and writing resources you need for your assignments. Start exploring! Subjectschevron_right ... Solved Label each carbon atom with the appropriate - Chegg Question: Label each carbon atom with the appropriate hybridization. This problem has been solved! See the answer ... Label each carbon atom with the appropriate geometry. CH2CH(CH2)2CCH Before overlapping with 1s orbital of hydrogen, first, the atomic orbitals of carbon undergoes hybridization to form hybrid orbitals. The hybrid orbitals of carbon involve in bond formation with hydrogen. Hence, the geometry at each carbon depends on the type of hybridization. Fundamentals The geometry of sp3 hybridized carbon atom is tetrahedral. Answered: of 20 > Label each carbon atom with the… | bartleby Transcribed Image Text: of 20 > Label each carbon atom with the appropriate hybridization. sp hybridized CH3 Answer Bank sp hybridized sp hybridized ECH, sp hybridized sp hybridized at sp hybridized CH, sp hybridized lo Tv sp hybridized ČH3 an wit pai PrtScn Home End F10 PgUp F8 F9 Expert Solution Want to see the full answer?
PDF The Hybridization Model of Atoms in Molecules - CPP The shape of the carbon atoms must be linear, because we know the hybridization is sp. C2H2 All of the details in this group go together. If you have any one of them, you should be able to fill in the remaining details. This is the ultimate in condensing a structure. OChem Spring 2017 Exam 1 Flashcards - Quizlet Label each carbon atom with the appropriate hybridization (cover bottom) ... Label each carbon atom wiht the appropriate geometry (cover right side) Sapling Hw Ch 1.21. Predict the molecular shape of methane, the carbonate ion, carbon dioxide, and the sulfite ion (cover left side of screen) Finding the hybridization of atoms in organic molecules ... Single bonded carbon is sp3 hybridized. Double bonded carbon is sp2 hybridized. Triple bonded carbon is sp hybridized. We get this from the formula for calculating the hybridization where -> Steric Number=No. of sigma bonds + No. of lone pairs ....and then then the value of the steric number gives us the number of hybridized orbitals. 19.png - Question 4 of 15 Solution 9 sapling\ufb01feaming ... View Homework Help - 19.png from CH 221 at North Carolina State University. Question 4 of 15 -/ Solution 9 saplingfifeaming Label each carbon atom with the appropriate hybridization. ! lsp3
What is the hybridization of the carbon atom labelled 2? It is s p hybrid because the labelled 2 carbon has C − C triple bond, and it has 2 sigma bonds, and zero lone pairs, hence, it has s p hybridization. Two sp hybridized orbitals: The chemical bonding in compounds such as alkynes with triple bonds is explained by s p hybridization. hybridization of cyclohexane The central atom is surrounded by two electron groups and is involved in two bonds, so it is sp hybridized. 1. the carbon-hydrogen bonds in cyclohexane are always eclipsed. All of the carbon atoms are in s p 3 hybridization forming a hexagonal network and the hydrogen atoms are bonded to carbon on both sides of the plane in an alternating manner. Label each carbon atom with the appropriate geometry. Label each carbon atom with the appropriate hybridization. sp,sp2 or sp3 Label each carbon atom with the appropriate hybridization. sp,sp2 or sp3 Posted one year ago Recent Questions in Chemistry Solved Label each carbon atom with the appropriate | Chegg.com Label each carbon atom with the appropriate hybridization. Question: Label each carbon atom with the appropriate hybridization. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer. Who are the experts?
8.2 Hybrid Atomic Orbitals - Chemistry Explain why a carbon atom cannot form five bonds using sp 3 d hybrid orbitals. What is the hybridization of the central atom in each of the following? (a) BeH 2 (b) SF 6 (c) PO 4 3− (d) PCl 5. A molecule with the formula AB 3 could have one of four different shapes. Give the shape and the hybridization of the central A atom for each.
Solved Label each carbon atom with the appropriate - Chegg Solved Label each carbon atom with the appropriate | Chegg.com. Science. Chemistry. Chemistry questions and answers. Label each carbon atom with the appropriate hybridization. sp,sp2 or sp3.
Can you label each carbon atom with the appropriate hybridization .... C=C bonded C atoms are planar and sp2 hybridized. C-C bonded C are sp3 hybridized. In this compound one C=C present and hence both carbons are sp2 hybridized. Left all the C atoms have four single bond around each C atom. so left all the C atoms are sp3 hybridized. number of electrons pairs around sp3 hybridized C atoms = 4 and
Hybridization - sp, sp2, sp3, sp3d, sp3d2 Hybridized ... All the compounds of carbon-containing a carbon-carbon double bond, Ethylene (C 2 H 4) sp 3 Hybridization When one 's' orbital and 3 'p' orbitals belonging to the same shell of an atom mix together to form four new equivalent orbital, the type of hybridization is called a tetrahedral hybridization or sp 3 .
What is the hybridization of each carbon in this molecule ... Explanation: This is because the first carbon has formed four bonds. So as you can see from the picture one electron from 2s orbital moves to the empty 2pz orbital. The 2s and the three 2p orbitals hybridise together and each orbital will be completed by adding one more electron from sharing with N, H, H, and the other C.
Chapter 9 Homework Flashcards - Questions and Answers - Quizlet There are 6 C atoms in the molecule. Starting on the left, the hybridizations are: sp2, sp2, sp3, sp, sp, sp3. All single bonds are bonds. Double and triple bonds each contain 1 bond. This molecule has 8 C-H bonds and 5 C-C bonds, for a total of 13 bonds. Double bonds have 1 bond and triple bonds have 2 bonds. This molecule has a total of 3 bonds.
OneClass: Label each carbon atom with the appropriate hybridization.sp ... Get the detailed answer: Label each carbon atom with the appropriate hybridization.sp,sp2 or sp3 Label each carbon atom with the appropriate hybridization.
Name the geometry around each carbon atom. What is the ... Below is the Lewis structure of cyclohexane (C 6 H 12) molecule, a cyclic compound used in the manufacture of nylon and found in the distillation of petroleum.. Name the geometry around each carbon atom. What is the hybridization of each carbon atom?



Post a Comment for "44 label each carbon atom with the appropriate hybridization."